The atom of an element consists of an electron, proton,
and neutron, do you know what is a proton and what is its charge. Things
around us are made up of different elements and every element has an atom that
consists of electrons, protons, and neutrons.
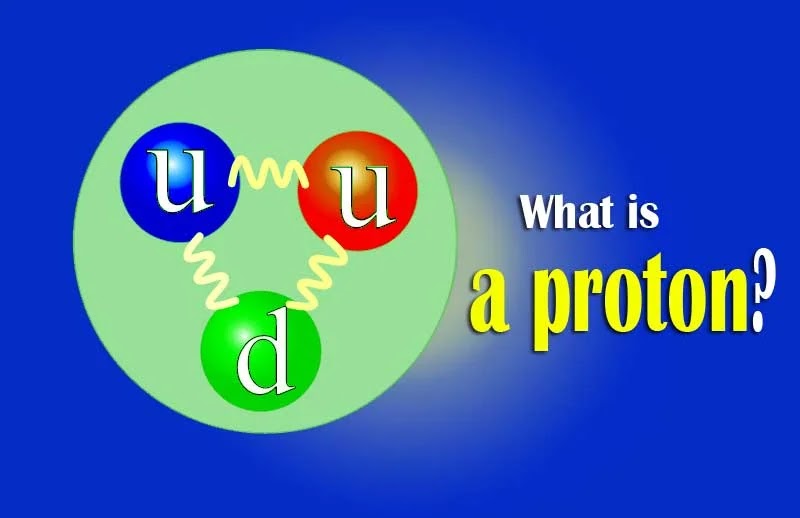 |
| What is a proton in atom |
In this article, we will know about what is a proton, what is its charge, when it was discovered, etc. So let's all know what is a proton.
What is a proton?
The proton is a basic subatomic particle with a positive
electrical charge. It is found in the atom with neutrons in the nucleus while
electrons revolve around the nucleus in Orbit. The word proton is Greek for
"first", and the name was given to the hydrogen nucleus in 1920 by Ernest
Rutherford.
Protons are fermions that have 1/2 rotation and are made up
of three quarks, i.e. they are in the form of baryon (a type of hadron). Their
two up-quarks and one down-quark would have been connected with a strong force.
The pairing of nuclei and neutrons is called nucleons, which are bound together
by nuclear force in the nuclear nucleus.
Hydrogen is the only element whose proton is found
alone in the atomic nucleus, otherwise, a proton is found with neutrons in the
nucleus of all other atoms.
Definition of Proton
Proton is a subatomic particle with a positive electrical
charge. Which are found with neutrons in the nucleus of an atom. It is
represented by a p + sign. The number of protons present in an element is
called the atomic number of that element. For example, suppose the number of
protons in an element is 20, then the atomic number of this element will also
be 20. Elements are placed in the periodic table based on atomic number.
What is the Mass of a proton?
The mass of a proton is 1.67262192369 × 10−27kg,
938.27208816 MeV / c or 1.007276466621 u, which is about 1847 times the electron's
mass.
Properties of protons
The proton is one of the three main particles of the atom.
The other two particles are neutrons and electrons. Protons inside the nucleus
of an atom help to bind the nucleus together. They also attract negatively
charged electrons and orbit them around the nucleus.
Protons consist of three primary particles, two up-quarks,
and one down-quark. Independently it is found as Hydrogen ion H +.
Its weight is slightly less than a neutron. It carries a (+
ve) charge of 1.6E-19 coulomb magnitude. Every Neutral nuclear has the same
number of electrons and protons.
Discovery of protons
The discovery of the proton is attributed to Ernest
Rutherford, who proved that the nucleus of a hydrogen atom (i.e. a proton) is
present in the nucleus of all other atoms in the year 1917.
Ernest Rutherford observed that his reflection detectors
detected hydrogen nuclei when a ray of alpha-particles was shot into the air.
Upon further investigation, Rutherford discovered that these
hydrogen nuclei originated from nitrogen atoms present in the atmosphere.
They then proceeded to fire beams of alpha particles in pure
nitrogen gas and saw that a greater number of hydrogen nuclei were produced.
He concluded that the hydrogen nucleus originated from the
nitrogen atom, proving that the hydrogen nucleus was a part of all other atoms.
This experiment was the first to report an atomic reaction,
given by the equation: 14N + α → 17O + p [where α is an alpha particle
consisting of two protons and two neutrons, and 'p' is a proton.
The hydrogen nucleus was later named 'proton' and was
recognized as one of the building blocks of the atomic nucleus.
see also: discovery of an electron
Conclusion: Proton is Fermion, which has spin 1/2, and it is
made up of three quarks. It occurs with neutrons inside the nucleus of an atom.
I sincerely hope that I have given you a lot of information
about what is a proton, as well as the discovery, mass, and properties
of protons. Please comment if you have any confusion. I request all of you
readers to share this information on various social media so that our awareness
remains and it will benefit everyone. I need your help so that I can give you
more new information. Please subscribe to our website.
Thank you!











0 Comments
If You need any new topic related post, please comment us.